Introduction
Cold agglutinin disease (CAD) is a serious and rare autoimmune disorder associated with severe fatigue, weakness and other symptoms of chronic anemia and hemolysis. Prior studies have demonstrated that CAD is associated with substantial economic burden and healthcare resource use, including blood transfusions, and highlight an unmet need for novel treatments (Mullins M et al. Blood Adv 2017; Su J et al. J Med Econ 2019). In the pivotal CARDINAL study (Part A), sutimlimab (BIVV009), a monoclonal antibody that targets the underlying cause of hemolysis in CAD by selectively inhibiting complement C1s, provided clinically meaningful improvements with an acceptable safety profile in CAD patients (Röth A et al. Blood 2019). A post hoc analysis was conducted to describe the impact of sutimlimab treatment on healthcare resource utilization (HCRU) in CAD patients.
Methods
CARDINAL is a Phase 3 open-label, international, multicenter, single-arm study (NCT03347396), evaluating the efficacy, safety, and tolerability of sutimlimab in patients with primary CAD and a recent history of blood transfusion (defined as having ≥1 transfusion 6 months prior to enrollment). The study enrolled 24 patients who received an IV infusion of sutimlimab over approximately 60 minutes on Day 0, Day 7, and every 14 days thereafter through Week 25. Post-hoc analysis of the full analysis set assessed hospitalizations and transfusions that occurred in the 6 month period pre- and post-administration of the first treatment dose. Distribution and descriptive statistics (mean, SD, median, min and max) were determined pre- and post-treatment periods and calculated for the differences between the pre- and post-treatment periods.
Results
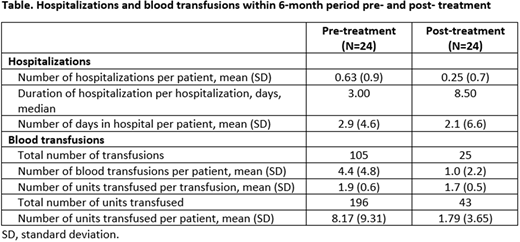
The number of hospitalizations experienced by CAD patients in this post hoc analysis decreased 3.3-fold following sutimlimab treatment: from 10 patients (41.7%) in the pre-treatment period to 3 patients (12.5%) in the post-treatment period. Pre-treatment hospitalizations were the result of CAD-related symptoms or blood transfusion. Reasons for post-treatment hospitalizations included sepsis, infection and blood transfusion. The mean number of hospitalizations per patient decreased post-treatment compared with pre-treatment, but the mean duration of hospitalization per patient was similar between treatment periods (Table). During the post-treatment period, one patient was hospitalized for 18 days due to an infected hematoma following surgery, unrelated to sutimlimab treatment. Nine patients (37.5%) experienced fewer hospitalizations, shortened mean and total hospitalization duration following treatment.
Similarly, blood transfusion use by CAD patients also decreased following sutimlimab treatment. Pre-treatment, all patients received ≥1 transfusion, decreasing to 41.7% patients post-treatment. This represented a total of 105 transfusions pre-treatment and 25 transfusions post-treatment, respectively (Table). Overall, 95.9% (23/24) patients received, at least, one less transfusion during the post-treatment versus pre-treatment period. The mean number of units transfused per transfusion was similar pre- and post-treatment; notably, the total number of units transfused decreased from 196 pre-treatment to 43 post-treatment (Table).
Conclusion
This post hoc analysis of HCRU data from the CARDINAL study provides evidence that CAD is a serious condition that can require hospitalization and blood transfusions. Sutimlimab treatment demonstrates substantial reductions in hospitalizations and blood transfusions. This results in a reduced HCRU impact and decreases the associated burden for hospitals, patients and payers.
Röth:Roche: Consultancy, Honoraria, Research Funding; Biocryst: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Research Funding. Guillonneau:Sanofi: Current Employment, Current equity holder in publicly-traded company. Narcisse:Sanofi: Consultancy. Carita:Sanofi: Current Employment, Current equity holder in publicly-traded company. Su:Sanofi: Current Employment, Current equity holder in publicly-traded company. Joly:Sanofi: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal